I am a biotechnologist who recently earned a Ph.D. degree in molecular biology and genetics. Although I have always worked with bacteria, I believe knowledge is transversal to the life sciences, through every level of complexity with the due scalability, never underestimating the power of learning from the small things. My research has always been focused on finding new alternatives to overcome current health challenges. Every day, I get the chance to observe that fiction and science are more alike than we might think. It is often only a matter of time.
A superhero’s tale
There are few people in the world who had never wished to be superheroes when they were kids. Even as adults, we sometimes wonder how much easier some aspects of our lives would be if we had super strength or X-ray vision.
A superhero who has some incredible—and rather odd—superpowers is Spiderman. We all know the story: a super smart, thin, and clumsy kid got bitten by a radioactive spider and became extra agile, strong, and athletic; was able to crawl on walls and ceilings; and gained the “spider sense.” None of those “powers” is new to nature. Anyone who has already seen a spider running away has witnessed the speed of average-sized, regular spiders. Studies have also demonstrated that some spider species can carry several times their weight, even when hanging from the ceiling. In addition, the ability to crawl on totally flat surfaces, like ceilings and walls, is well known to all of us.
Now, imagine that amplified by radiation and then, somehow, transmitted to a human being with his or her corresponding proportions, and you get Spiderman’s powers. Even the Spiderman spider sense probably results from an overamplified capacity to feel vibrations, which, in the case of spiders, is due to tiny hairs and slits distributed all over their bodies. Web-slingers apart, all those superhuman abilities were given to Peter by the spider. But how?
The genetics of Spiderman
We can only infer the mechanisms involved in this strange transference of traits, as no information was provided by the creators of Spiderman. By biting Peter Parker, the spider injected some of its radioactive poison into Peter’s bloodstream. Luckily, he did not suffer any anaphylactic shock, and his body seemed to adapt well to the presence of the spider’s poison—so well that he even evolved to something new. The radioactive poison induced Peter’s cells to change their metabolism within the whole body—his skin, muscles, and even brain.
By the alteration of the genetic code, it is possible—although improbable at that extent. Somehow, the poison could induce specific mutations in Peter’s DNA. Alternatively, the poison itself could contain the spider’s DNA, which was recombined with Peter’s human DNA, allowing the expression of spider traits. DNA recombination is a natural process that involves the exchange of genetic material among multiple chromosomes or between different regions of the same chromosome in a cell. It is responsible for increasing the variability within eukaryotic species.
Here, something different happens—the production of recombinant DNA, which may include sequences from distinct organisms. Although this does not occur naturally in eukaryotes, it happens in bacteria that accommodate exogenous DNA—i.e., DNA from other species—into their genomes under specific circumstances. Also, it can be artificially induced in a laboratory.
There are several known mutagenic agents (that is, they can induce changes in a DNA sequence), including chemical compounds, biological agents (perhaps spider poison), and radiation. In most cases, humans can repair these mutations, which are usually identified as errors in the DNA sequence, and the cells keep their regular functions.
Otherwise, cells can undergo apoptosis, which is also referred to as programmed cell death. This is the mechanism used by the body to get rid of cells that are damaged beyond repair, namely, due to the accumulation of errors in the DNA. This triggers a cascade of reactions, starting with the signaling of the abnormal cell, continuation to shrinking and DNA fragmentation processes, and finishing with elimination of the cell. If the errors cannot be repaired and start accumulating, and the apoptosis process is somehow impaired, this leads to uncontrolled cell division and the subsequent development of cancer.
Some mutations can be tolerable, as they can be
- silent, which means that the final product of that gene will not be affected
- in a region that does not encode relevant information
- simply ignored by the activation of DNA damage tolerance pathways, which allow the DNA replication to continue, bypassing the damaged region.
Therefore, mutations can be irreversible and heritable; however, mutations are not necessarily bad. The existence of a low rate of mutations introduces some variability within the genome of a given species and, consequently, individual organisms’ characteristics. That is particularly important to increase the adaptability of the species as a whole.
In this regard, Charles Darwin published On the Origin of Species (1859), in which he proposed the theory of evolution by natural selection. Briefly, this theory postulates that evolution is driven by the selection and survival of the fittest organisms, meaning those that are most suited for the environment where they live. These will be more likely to reproduce and, therefore, pass their traits on to the next generation. This also means that, if the environment changes, the selected traits will also gradually change—or evolve. Therefore, specific environmental conditions can sometimes favor a particular characteristic that initially was present in only a small part of the population.
A common example is sickle cell anemia. This is a genetic disorder characterized by the production of abnormal red blood cells, which are less efficient at transporting oxygen and flowing within the bloodstream. However, this condition brings about an unexpected advantage to people infected with malaria. It seems that individuals carrying one copy of the mutated gene that is responsible for sickle cell anemia are highly resistant to malaria.
This vector-borne, infectious disease is caused by a parasite, Plasmodium falciparum, which is transmitted by mosquitos. When sickle cells are infected with this parasite, they collapse, which prevents the parasite from interfering with other relevant proteins within the cell, thereby protecting the host against malaria. Therefore, this gene is particularly recurrent in areas with a high incidence of malaria, such as central Africa and central America, as people carrying it are more likely to survive in those regions.
Back to our superhero: in the case of Peter Parker, mutations induced by the poison—by means of whatever mechanism—made him Spiderman. However, one main question emerges: is that possible, scientifically speaking? If so, how far are we from being able to do it?
To start with, scientists would definitely not use spiders to inject whatever they decided would be required. Lots of people are afraid of needles, but they are still preferable to spiders or any other biting, stinging, or touching animal. However, the delivery method is the last part of this intricate puzzle. The real deal is to develop a mechanism for the introduction of the mutations, per se, within the genome. The truth is that this is already being done in many laboratories around the world, just not in humans (yet—or, at least, not permanently).
Manipulating genes for the greater good
First of all, scientists have been inducing mutations for a long time. To understand this, we have to remember that humans have been trying to manipulate evolution since the very beginning.
This is particularly obvious when we look, for instance, at crops like maize, wheat, and rice, which are currently used for feeding, and compare them to those from several years ago. We have selected the biggest ones—those that produces more grain—and kept their seeds, hoping that, the following year, they would grow even larger and be more productive. At some point, variants were lost, and the ones remaining are best fitted to the climate we are living in. We also try to get the best out of our animals by choosing those with some desirable characteristics, and we keep doing that in the following generations.
However, all of these attempts to manipulate evolution raise ethical issues concerning how far we should go and if the end justifies all of the means. The problem of choosing specific traits is that, when something in the environment changes, the selected characteristics may not be desirable anymore. Until not long ago, we would have to go out there again, look for other variants and repeat the process. However, at some point, scientists realized that there should be a faster and more efficient way.
With their increasing knowledge of genetics as well as the evolution of molecular biology techniques and other disciplines in the life sciences, scientists learned how to read the information contained within DNA. Furthermore, they learned that it could be “copied and pasted.” One can imagine how difficult it is to read human DNA. Its whole extent is estimated to be around 2 m long and carry the equivalent of 1.5 Gb of information (considering the four-letter code in which it is written) in a single cell. It is possible to literally “read” a genome by sequencing technologies—particularly, whole-genome sequencing.
In simple words, DNA sequencing is used to determine the exact sequence of a DNA molecule, using the mentioned four-letter code. After finding the sequence, this must be “translated”; i.e., the sequence is divided into “words” and “sentences” that will give origin to the amino acids and proteins that build up a body. Then, it is possible to compare the sequences of different organisms and observe the singularities between individuals from the same species.
Whole-genome sequencing refers to a comprehensive method for analyzing the complete DNA sequence of an individual organism. DNA-sequencing methodologies have been evolving since the 1970s to become faster, more automated, and cheaper. The first organism whose genome was sequenced was the bacterial species Haemophilus influenzae. However, the Human Genome Project was the biggest booster of these technologies; the first human whole-genome sequencing was announced in 2003 and is being continuously updated today.
It is not a coincidence that the first-ever sequenced genome was a bacterial one. Scientists have started with far simpler organisms: bacteria. In this context, simpler only means that their genomes are far smaller than those from eukaryotes, and thus making them easier to read.
Genetic engineering was born in the second half of the 20th century with the discovery of restriction enzymes (natural proteins with the ability to cut DNA in specific sequences), which provided tools that allow DNA manipulation. A few years later, in 1973, two biochemists, Stanley N. Cohen and Herbert W. Boyer, were able to cut DNA fragments and then merge and insert them within a bacterial genome. Then, the proteins corresponding to the inserted DNA were produced by those bacteria.
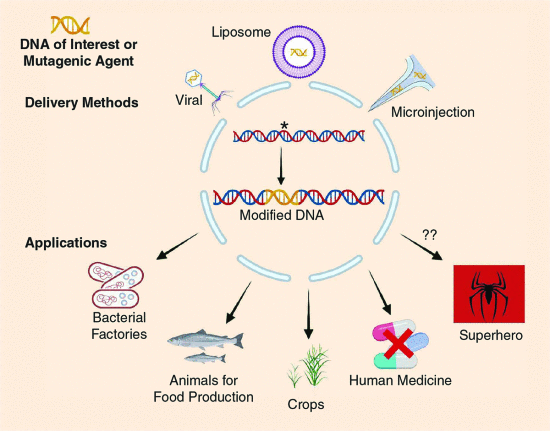
Although some requirements must be met, and optimizations are often required, this approach has been successfully applied to create bacterial factories that are used to synthesize human insulin, human growth hormone, alpha interferon, and a hepatitis B vaccine, among other medically useful substances (Fig. 1). Bacteria and yeasts are currently used to express and study the effects of specific mutations in proteins from diverse origins, contributing to improving our understanding of several practical and theoretical aspects of gene function and organization.
Fig 1Genetic manipulation and gene therapy. A brief overview of the main techniques used to insert or induce changes in DNA and possible applications. Created with https://biorender.com.
Knowledge increases exponentially. Regarding more complex organisms, plants can also be genetically modified. The most wanted characteristics are mostly related to
- resistance to genetic diseases, plagues, or drought
- the enrichment of their nutritional value, mainly to be cultivated in developing countries
- enabling nitrogen fixation.
Nevertheless, special attention must be given to possible unwanted side effects: the more the complexity of the organism increases, the more intricate the crosstalk among several genes becomes. Manipulating a gene involved in a certain trait can have an unexpected impact on another cascade, which may result in unwanted, and even harmful, traits.
It should come as no surprise that animals have also been genetically engineered. Known and controversial examples are, for instance, salmon, which have been engineered to grow larger and faster; cattle that were enhanced to become resistant to mad cow disease in the United States; and animals for laboratorial applications, such as mice. Naturally, as our ability to precisely engineer and edit animals’ genomes increases, the public concern and ethical issues rises to the same extent, despite the intended potential benefits.
Regarding both animals and plants, there is a major drawback that is absent when dealing with bacteria: they are multicellular. In bacteria, one can add the desired DNA to a bacterial suspension and, with a rather simple protocol, ensure the insertion of such DNA in a significant number of bacterial cells that can be further propagated. In more complex organisms, scientists deal with several aspects when trying to induce the required mutations—the main one being their complexity.
Altering the genome of a multicellular organism is not a precise science since unexpected outcomes may arise, depending on the targeted cells. Multicellular organisms are built up by several types of differentiated cells that, although sharing the same genetic code, express different genes and have the most diverse and complementary functions.
It is important to distinguish between two groups of cells: germ and somatic lines. Germ cells give rise to the gametes of an organism and originate from the primitive streak of the embryo; somatic cells are basically all of the other cells that are not from the germline and constitute the whole body.
An important aspect is that mutations in somatic cells will affect only the individual in which the gene manipulation is conducted and will not pass through generations, not altering the evolution of the species. Quite the contrary, mutated germ cells will give rise to mutated gametes that will pass the mutations to the following generation via sexual reproduction. Although the genetic manipulation of somatic cells is currently used and more easily accepted in therapies associated with several diseases—namely, some types of cancer—the manipulation of the germline is highly controversial and is allowed only for research purposes.
Another barrier to the manipulation of cells from more complex organisms is related to the required methodology to mutate their genomic information. Again, nature provides answers about the most suitable tool with which to introduce DNA into a host cell and force it to produce proteins that were not coded there before: viruses. These are not even considered “beings,” as they cannot survive by themselves without a host to infect. However, they are perfectly equipped to deliver genetic information into a complex organism.
Scientists started engineering viruses, making them not harmful by impairing their replication while maintaining their ability to deliver genetic material with the required information that would then translate into desirable characteristics. For example, this approach was successfully applied in gene delivery in plants to induce desirable agronomic traits or produce valuable biotechnological compounds, including pigments and vaccines.
Another well-known example is Dolly the sheep, the first-ever cloned adult mammal, born in 1996. British developmental biologists from the Roslin Institute (Edinburgh, Scotland) cloned a somatic cell from a mammary gland, taken from an adult ewe, and used electrical pulses to fuse it with an unfertilized egg cell whose nucleus had been removed, which then began to divide. This constituted a milestone since it proved that adult mammals could be successfully cloned using somatic cells.
Following this success, the consequent debate concerning the many possible uses and misuses of mammalian cloning technology was ignited. Other approaches can be employed to deliver foreign DNA to a new host, and those are discussed in the following section, with a particular emphasis on potential human hosts.
How about humans?
Can we actually “edit” humans? Reported successes in the correction of genetic errors associated with disease in animals suggests a potential application of gene editing in gene therapy for humans. Currently, there are several trial studies aiming to apply gene therapy to overcome some disorders, including several types of cancer, AIDS, cystic fibrosis, hemophilia B, and rheumatoid arthritis, among many others. However, a lot is still required to make this therapy available on a regular basis since the process is very complex, and efficient techniques must be developed and optimized case by case—sometimes, patient by patient.
First, it is important to deeply understand the targeted diseases and their genetic bases as well as possible interactions with other cells/organs/systems. Then, it is equally essential to identify which cells are specifically affected and understand how it would be possible to reach them. Since all human cells carry the same genetic information, altering the whole genome often is not the right solution.
Gene therapy can be designed to be applied in either stem or somatic cells. As mentioned, mutating stem cells, such as a fertilized egg, would allow the substitution of a defective gene by integrating a functional gene into the genome. These alterations would be extended to all of the individual’s cells, and, consequently, they are hereditary and transferable to subsequent generations, thereby mitigating genetic and hereditary diseases. As discussed, for ethical reasons, germ cell editing is allowed only for research purposes. On the contrary, by applying gene therapy directly to somatic cells, only the specifically targeted cells would be affected. Those effects would be restricted to the patient and would not be inherited by future generations.
The method by which the correct DNA sequence is delivered within the targeted cells is a critical step for a successful implementation of gene therapy. When dealing with human medicine, several aspects must be considered, starting with safety concerns for the patient, environment, and professionals who manipulate it. The vehicle—the so-called “vector”—must be very specific while showing high efficacy to release the desired DNA. Additionally, it should not induce allergic or inflammatory responses in the patient’s immune system. Upon delivery, the newly added DNA is expected to do one of the following: increase normal functions, correct deficiencies, or inhibit deleterious activities.
The industrial feasibility of the production of large amounts of the vector is not of less importance. The techniques currently under consideration are divided into two major groups: virus-mediated and physical mechanisms, which include several approaches, from cationic polymers and liposomes to DNA microinjections and particle bombardment. The adequate mechanism must be chosen according to the nature of the DNA to be inserted and specific application.
In the last few years, several therapies have been approved for use in human medicine after decades of efforts. These are to be applied in the treatment of several clinical conditions, including neuromuscular diseases, inherited blindness, and cancer, bringing new hope to patients whose diseases were considered uncurable or whose symptoms were difficult to experience and overcome.
Once we have enough knowledge regarding the specific function of each human gene and the interactions among genes, it would definitely be possible to manipulate virtually any trait that is encoded within our genome. Currently, genetic screening is used to select embryos generated via in vitro fertilization. A preimplantation genetic diagnosis is run in a single cell from the eight-cell embryo, whose DNA is analyzed for the presence of diseases associated with genetic alterations before the implantation in the mother.
This screening used to be performed only to determine the sex of the embryos to avoid the transmission of sex-linked diseases that could be identified in families’ medical histories. Since then, the genetic diagnosis of embryos has been applied to detect single-gene-related diseases, such as Huntington’s disease, and it is now used to diagnose more than 170 different conditions, including cystic fibrosis and hemoglobin disorders. The screening can also be applied to detect chromosomal abnormalities in an attempt to improve pregnancy rates and decrease the levels of miscarriage associated with in vitro fertilization. Recently, genetic screening was also employed to identify—and select—embryos not carrying genes associated with increased breast cancer risk to irradicate breast cancer in families who have been suffering from this condition for several generations.
However, each of these advances raises more controversy around the application of gene-based selection. Likewise, the application of gene editing in humans raises ethical concerns, particularly regarding its potential use to alter other traits that go beyond health issues. As in all scientific fields, this is full of steps back and forward.
Among unsuccessful trials, one can list the gene therapy trials conducted in France (2002) with children suffering from severe combined immune deficiency (SCID), a disease that is linked to the X chromosome. Although the first results seemed promising, with general improvement of the children’s condition, after a few months, some of them started showing signs of cancer-like diseases that were likely a direct consequence of the treatment. More recently, a scandal involving the birth of twin girls with allegedly edited genomes (2018) brought this issue to the spotlight, with the World Health Organization proposing the formation of an international committee to establish strict guidelines for human gene editing.
On the other hand, the first gene therapy success story occurred in 1990 with a four-year-old girl who also suffered from SCID. Currently, there are nearly 400 active gene therapy trials around the world, and an increasing number of gene therapy drugs are starting to enter the market.
Ethics in genetics
“With great power comes great responsibility”: while we may have—or may acquire—the skills to perform gene editing, should we do it without limits? Once we can identify the right “switches,” we should be able to create “superplants” and “superanimals”—or even “superhumans” who are immune to a wide range of diseases, stronger, more intelligent, and maybe prettier. Why not? At some point, parents would even decide the physical aspects and traits of their child to make “the perfect baby”.
Sceptics ask if we should alter our “instruction manual” indiscriminately, even if, or when, we have the expertise to do it. Perhaps, at some point, this kind of manipulation could be used to achieve specific external characteristics with no relation to health outcomes. Although most of these traits are often subjective and might depend on variable trends and fashion moods, we must consider the role of variability in evolution. As discussed, it is variability that provides a species with a full toolbox of possibilities to adapt to any upcoming event. As we select and refine specific characteristics, other traits will naturally be lost over time, and these might be useful one day, later in our evolution as a species.
In conclusion
To conclude with a short answer to the question that drove us here: is it possible for a human to become a Spiderman? Scientifically speaking, with the exponentially growing knowledge in genetic manipulation and insights into the human genome, we can be sure that it will be possible to control genetically encoded traits toward any desired characteristic. However, we are not there yet. The mechanisms needed to induce mutations and substitute genes still require extensive investigation, and the exact effects of each shift need to be assessed and deeply characterized. Nonetheless, science is surely on the right path to make it happen, perhaps in the near future.
However, the main question remains: once we can do it, should we do it in any circumstance? To what extent? Ethical concerns should be carefully analyzed, as they raise valuable questions on the limits that should be imposed in the application of genetic manipulation approaches in humans in the future.
Although gene therapy products and research are strictly regulated now, as our knowledge increases, novel possibilities and problems may arise, requiring the solid establishment of new regulations to ensure that manipulation will not be used for less crucial—or even deleterious—purposes. In the meantime, we should always try to be informed about the pros and cons of such technologies and draw our own informed conclusions as far as this matter is concerned.